CHEMICAL AND PHARMACEUTICAL INDUSTRY
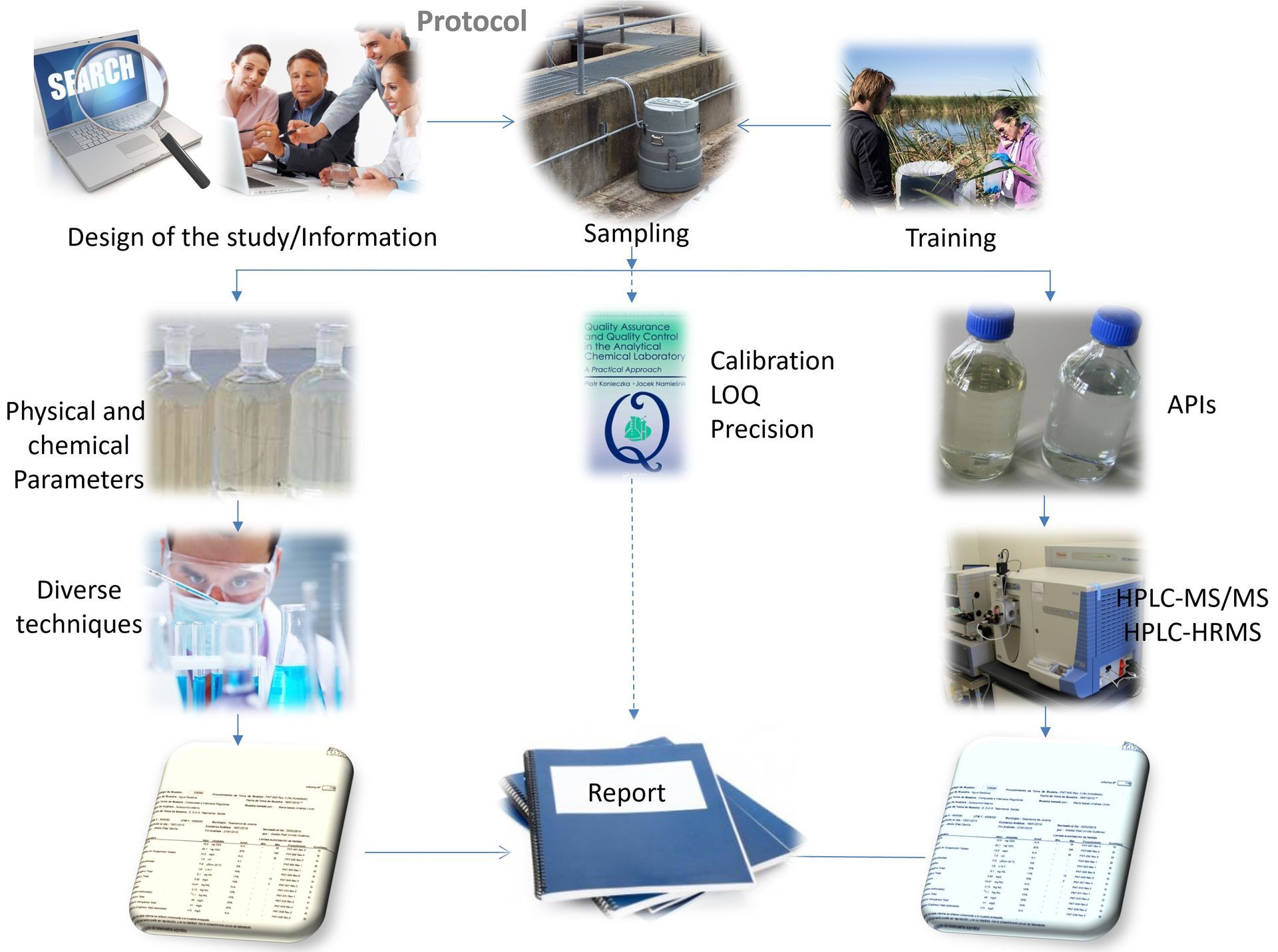
Wastewater studies (API)
In recent years, there has been increasing attention regarding the presence and effects of active pharmaceutical ingredients (API) in the different compartments of the aquatic environment. In fact, some of them are already on the EU "Watch-List" as well as in the most demanding monitoring regulations, such as the USEPA 1694 method, and their monitoring the discharges of manufacturing plants is starting to be required.
With the aim of addressing the minimization of the polluting load of the discharges in the short-medium term, it is convenient to reliably know the composition and levels of the APIs and by-products present in the waste water of the manufacturing plants.
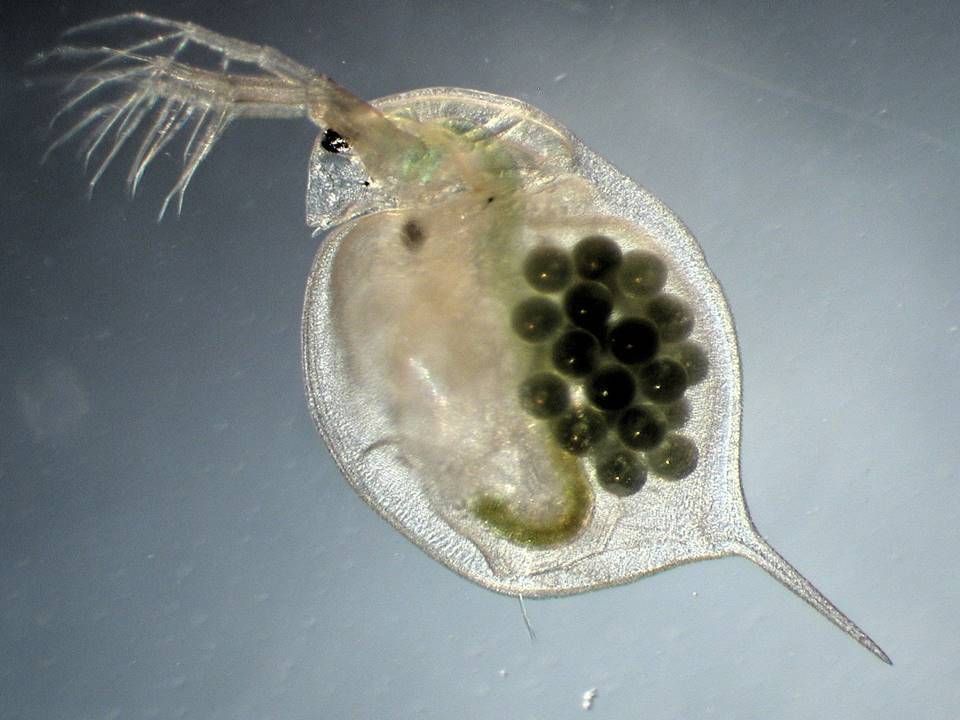
ERA of medicinal products. ECOTOX studies
Our technicians have extensive experience in the carrying out of Environmental Risk Assessment studies for human and veterinary medicines, according to the criteria of the European Medicines Agency.
Waste water purification projects of the pharmaceutical industry
It has been reliably verified, both in urban wastewater treatment plants and in those of the pharmaceutical plants, that conventional purification systems, in general, offer limited efficiencies for many APIs.
Consequently, in new production facilities, and when modernization projects of the existing WWTPs are carried out, it will be necessary to contemplate new designs of these infrastructures, adding the possibility of applying supplementary or alternative techniques in addition to those adopted until now.
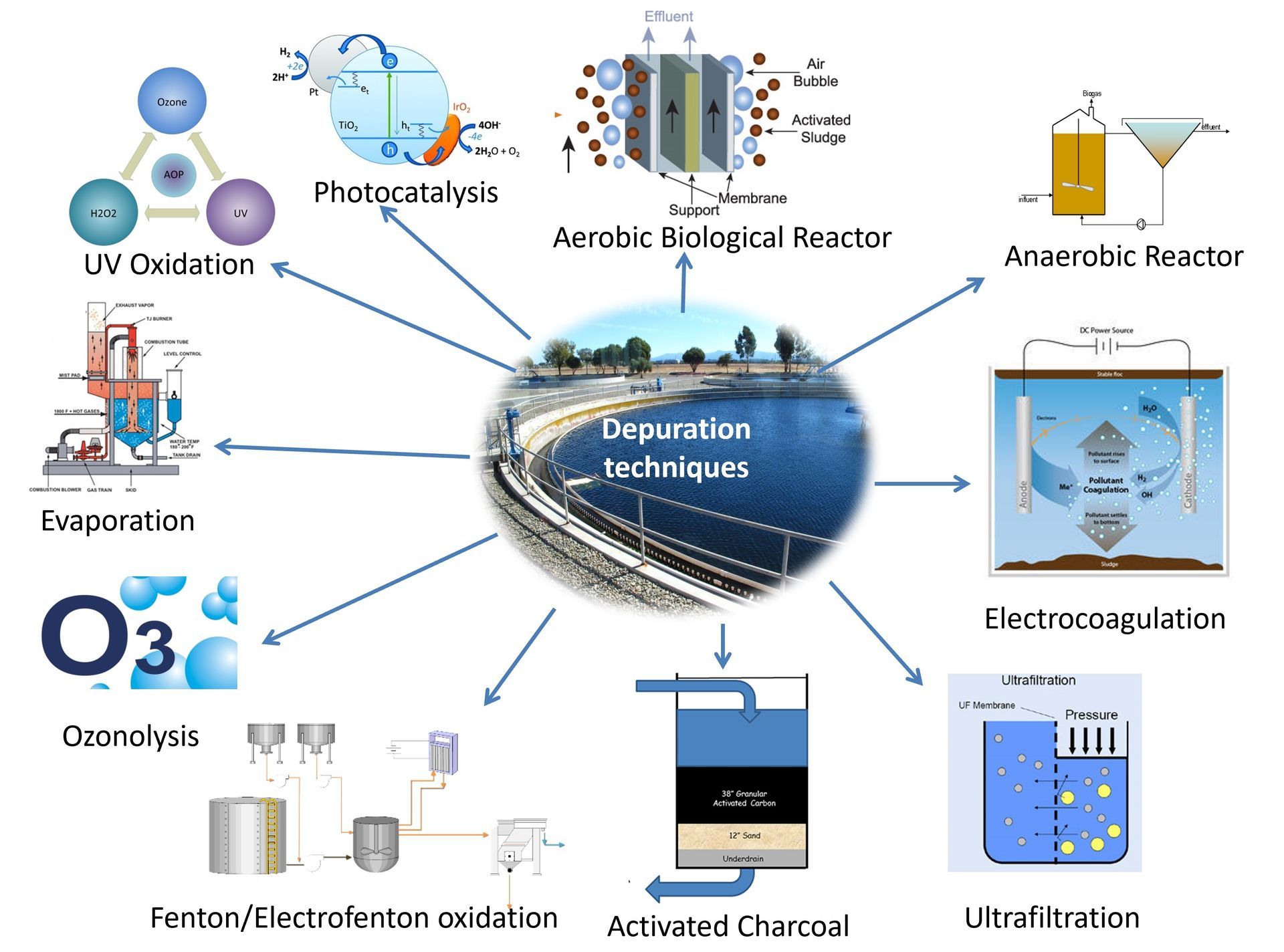
Range of waste water treatment techniques for the purification of drugs




